Nano-Plastics
Boiled Tap Water Reduces Human Intake of Nano-Plastics and Micro-Plastics.
Two Articles (by GreenMedInfo & Dr. Ana Maria)
“Boiling Away the Microplastics: A Simple Solution to a Complex Problem” – Article by GreenMedInfo:
https://greenmedinfo.com/content/boiling-away-microplastics-simple-solution-complex-problem
![]()
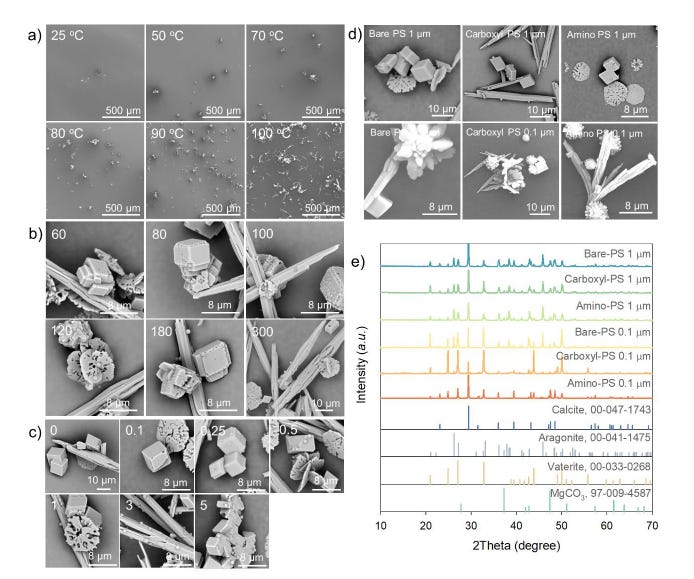
Morphology and composition of incrustants in different conditions. (a) Scanning electron microscopic (SEM) images of bare-polystyrene (PS, 1 μm, 1 mg L–1) and incrustant coprecipitates formed in tap water at different temperatures (180 mg L–1 of CaCO3, 40 mL, 25–100 oC); (b) SEM images of bare-PS (1 μm, 1 mg L–1) and incrustant coprecipitates in different water hardness upon boiling (60–300 mg L–1 of CaCO3, 100 oC); (c) SEM images of bare-PS and incrustant coprecipitates in different PS concentrations (1 μm, 0–5 mg L–1) upon boiling of tap water (180 mg L–1 of CaCO3, 100 oC); and (d) SEM images and (e) X-ray diffraction patterns of bare-, carboxyl-, and amino-PS and incrustant coprecipitates upon boiling of tap water (1 and 0.1 μm, 1 mg L–1, 180 mg L–1 of CaCO3, 100 oC).
͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏ ͏
Drinking Boiled Tap Water Reduces Human Intake of Nanoplastics and Microplastics
Morphology and composition of incrustants in different conditions. (a) Scanning electron microscopic (SEM) images of bare-polystyrene (PS, 1 μm, 1 mg L–1) and incrustant coprecipitates formed in tap water at different temperatures (180 mg L–1 of CaCO3, 40 mL, 25–100 oC); (b) SEM images of bare-PS (1 μm, 1 mg L–1) and incrustant coprecipitates in different water hardness upon boiling (60–300 mg L–1 of CaCO3, 100 oC); (c) SEM images of bare-PS and incrustant coprecipitates in different PS concentrations (1 μm, 0–5 mg L–1) upon boiling of tap water (180 mg L–1 of CaCO3, 100 oC); and (d) SEM images and (e) X-ray diffraction patterns of bare-, carboxyl-, and amino-PS and incrustant coprecipitates upon boiling of tap water (1 and 0.1 μm, 1 mg L–1, 180 mg L–1 of CaCO3, 100 oC). |